Chyros' chemistry thread
 Chyros
30 Jan 2011
Chyros
30 Jan 2011
The Rotavap
The rotavap is a common piece of machinery used to gently boil off solvents under reduced pressure. Using a membrane pump to create a vacuum will make solvents boil off faster, and rotating the flask gives you the advantage of creating more area for the solvent to evaporate off from as well as preventing superheating (which will result in the solvent suddenly boiling extremely violently). The rotavap combines all these with the option of using a water bath to keep the flask on room temperature (normally evaporation of solvents inside will quickly cool down the flask since evaporation is an endothermic process) or allows heating to speed up evaporation.
If you contaminate the rotavap with solvents that are hard to get rid of (such as water) or traces of compound (for example low-boiling organic compounds), you need to clean it to prevent reflux of these materials to contaminate the compounds of whoever uses the rotavap next. To do so, you can disassemble the machine and very manually clean it but this takes a disproportionate amount of effort, or you can use a splashing tube which is a bent glass tube with a socket at the end. If you connect this to the rotavap, fill it up with some clean solvent that evaporates easily, such as acetone, put on vacuum and block then end of the tube, the pressure in the rotavap will lower, and if you remove the blockade, the outside air will quickly rush in and push the solvent through the system, splashing it clean in an easy and convenient way.
I hate how nasal my voice sounds in these videos >.> .
The rotavap is a common piece of machinery used to gently boil off solvents under reduced pressure. Using a membrane pump to create a vacuum will make solvents boil off faster, and rotating the flask gives you the advantage of creating more area for the solvent to evaporate off from as well as preventing superheating (which will result in the solvent suddenly boiling extremely violently). The rotavap combines all these with the option of using a water bath to keep the flask on room temperature (normally evaporation of solvents inside will quickly cool down the flask since evaporation is an endothermic process) or allows heating to speed up evaporation.
If you contaminate the rotavap with solvents that are hard to get rid of (such as water) or traces of compound (for example low-boiling organic compounds), you need to clean it to prevent reflux of these materials to contaminate the compounds of whoever uses the rotavap next. To do so, you can disassemble the machine and very manually clean it but this takes a disproportionate amount of effort, or you can use a splashing tube which is a bent glass tube with a socket at the end. If you connect this to the rotavap, fill it up with some clean solvent that evaporates easily, such as acetone, put on vacuum and block then end of the tube, the pressure in the rotavap will lower, and if you remove the blockade, the outside air will quickly rush in and push the solvent through the system, splashing it clean in an easy and convenient way.
I hate how nasal my voice sounds in these videos >.> .
 Ghostrider
30 Jan 2011
Ghostrider
30 Jan 2011
Cool apparatus! Good thing you didn't turn on the rotor when you had the cleaning tube put on, lol. 
 Destiny
30 Jan 2011
Destiny
30 Jan 2011
They way you pulled out that flask from nowhere and kept it looked just like in FPSes
 Chyros
30 Jan 2011
Chyros
30 Jan 2011
 Ghostrider, on 30 Jan 2011, 14:08, said:
Ghostrider, on 30 Jan 2011, 14:08, said:
Cool apparatus! Good thing you didn't turn on the rotor when you had the cleaning tube put on, lol. 
 Destiny, on 30 Jan 2011, 14:15, said:
Destiny, on 30 Jan 2011, 14:15, said:
Quote
They way you pulled out that flask from nowhere and kept it looked just like in FPSes  Maybe you have the first person shooter disorder
Maybe you have the first person shooter disorder 
 Chyros
09 Jul 2011
Chyros
09 Jul 2011
Well, it's been a long while, because I haven't done lab work in quite a while (moving the lab, then writing thesis and whatnot) but now I'm back in it. I had a bunch of videos I made back in the days when I was still in the old building but decided to scrap most of them. I still have one though, of making a liquid nitrogen-methanol bath.
Synthesis sometimes needs to be conducted under reduced temperature. This can be because, for example, the reaction may proceed too fast to be selective at room temperature, or it may be too vigorous or exothermic and explode. As such, many studied reactions are optimised to a certain temperature.
The three most easily attainable temperatures are 0 deg C (cool with ice), -15 C (ice in salt water), -80 C (dry ice) and -200 C (liquid nitrogen). However, sometimes you need a temperature that may not be very straightforward to maintain, such as -100 C. Using certain liquids provides a way to maintain temperatures like this - in the case of -100 C, it is liquid nitrogen in methanol, as is what happens here. Methanol has a melting point very close to -100 C and liquid nitrogen is cold enough to get it to that temperature.
The methanol bath is put into place, and a dewar flask with liquid nitrogen is poured out slowly into it. As the liquid nitrogen comes closer to the surface of the (room temperature and therefore over 200 degrees hotter) methanol, the nitrogen starts to boil violently, splashing methanol everywhere. The nitrogen evaporates so fast that it will create a cushion on nitrogenous steam which prevents the nitrogen from touching the methanol for a while (this is called the Leidenfrost effect and it's also the reason you can touch liquid nitrogen or "drink" it - the temperature of your body is enough to evaporate it before it touches you and shield you from the liquids) and you can see this as a bubbling mass on top of the methanol. After a while, the methanol has lost so much of its heat to the nitrogen that it can't boil the nitrogen very fast anymore, so the nitrogen will start touching the methanol's surface. You can recognise this by the steam becoming less dense and less white. It will then form small lumps of solid methanol, and some stirring will then make everything solidify instantly.
Synthesis sometimes needs to be conducted under reduced temperature. This can be because, for example, the reaction may proceed too fast to be selective at room temperature, or it may be too vigorous or exothermic and explode. As such, many studied reactions are optimised to a certain temperature.
The three most easily attainable temperatures are 0 deg C (cool with ice), -15 C (ice in salt water), -80 C (dry ice) and -200 C (liquid nitrogen). However, sometimes you need a temperature that may not be very straightforward to maintain, such as -100 C. Using certain liquids provides a way to maintain temperatures like this - in the case of -100 C, it is liquid nitrogen in methanol, as is what happens here. Methanol has a melting point very close to -100 C and liquid nitrogen is cold enough to get it to that temperature.
The methanol bath is put into place, and a dewar flask with liquid nitrogen is poured out slowly into it. As the liquid nitrogen comes closer to the surface of the (room temperature and therefore over 200 degrees hotter) methanol, the nitrogen starts to boil violently, splashing methanol everywhere. The nitrogen evaporates so fast that it will create a cushion on nitrogenous steam which prevents the nitrogen from touching the methanol for a while (this is called the Leidenfrost effect and it's also the reason you can touch liquid nitrogen or "drink" it - the temperature of your body is enough to evaporate it before it touches you and shield you from the liquids) and you can see this as a bubbling mass on top of the methanol. After a while, the methanol has lost so much of its heat to the nitrogen that it can't boil the nitrogen very fast anymore, so the nitrogen will start touching the methanol's surface. You can recognise this by the steam becoming less dense and less white. It will then form small lumps of solid methanol, and some stirring will then make everything solidify instantly.
 Destiny
09 Jul 2011
Destiny
09 Jul 2011
I like the 'flash freeze' after stirring it, it's like being supercooled fishes under a layer of ice under a lake. Even more is all the mist being produced, it seems so...alluring.
 Chyros
09 Jul 2011
Chyros
09 Jul 2011
Oh, chemistry (synthetic organic chemistry at least) is full of interesting little visuals like that.
With a bit of luck I'll do an interesting distillation soon which I predict will produce a dense, intensely blue foam .
.
With a bit of luck I'll do an interesting distillation soon which I predict will produce a dense, intensely blue foam
 Alias
10 Jul 2011
Alias
10 Jul 2011
I never knew it until today, but now I know.
What was missing from my life has been dense, blue foam.
Edited by Alias, 10 July 2011 - 00:01.
What was missing from my life has been dense, blue foam.
Edited by Alias, 10 July 2011 - 00:01.
 Chyros
10 Jul 2011
Chyros
10 Jul 2011
 Chyros
10 Jul 2011
Chyros
10 Jul 2011
What the hell would we do with that?! 
We do have ultra-pure silica, though. The material it's made out of.
We do have ultra-pure silica, though. The material it's made out of.
 Destiny
10 Jul 2011
Destiny
10 Jul 2011
You could just...fool around with it I suppose, put it over your oxy-acetylene torch and say 'hey guys look I'm fireman' 
 Chyros
03 Aug 2011
Chyros
03 Aug 2011
 Destiny, on 03 August 2011 - 21:50, said:
Destiny, on 03 August 2011 - 21:50, said:
Makes you wanna smudge it all over someone's face/new shirt.
So you'd definitely have to buy a new shirt for them
I wasted about three shirts during my first year by simply being around it without proper lab coat protection - small amounts of vapours are enough to inconspicuously make small holes in your clothes everywhere until you take a look at your clothes and find they are actually completely falling apart.
Edited by Chyros, 03 August 2011 - 22:34.
 Chyros
04 Aug 2011
Chyros
04 Aug 2011
 Wizard
04 Aug 2011
Wizard
04 Aug 2011
 Chyros
04 Aug 2011
Chyros
04 Aug 2011
 Wizard, on 04 August 2011 - 07:44, said:
Wizard, on 04 August 2011 - 07:44, said:
Well actually the quality of lab coats differ enourmously. Pharmacists, biologists and the more theoretical types of chemist (and physicists if they dare enter a lab sometime
 Chyros
26 May 2013
Chyros
26 May 2013
Figured I'd revive this again, sharing some of the many lab pictures I've taken over the years. I'll just be posting a short story or a picture every time I do this, to give a flavour of what synthetic chemistry is all about.
Today; a part of my internship. I was making a series of organic polymers which I prepared in a copper solution.
The setup was like this:

The setup to the right (with the mechanical stirrer down the middle and the reflux bulb on the left) was used for the preparation of the crude product, the middle setup (with the makeshift infusion setup and massive conical flask) was used for aqueous workup and the one on the left (with the dropping funnel and reflux condenser) was for co-polymerisation.
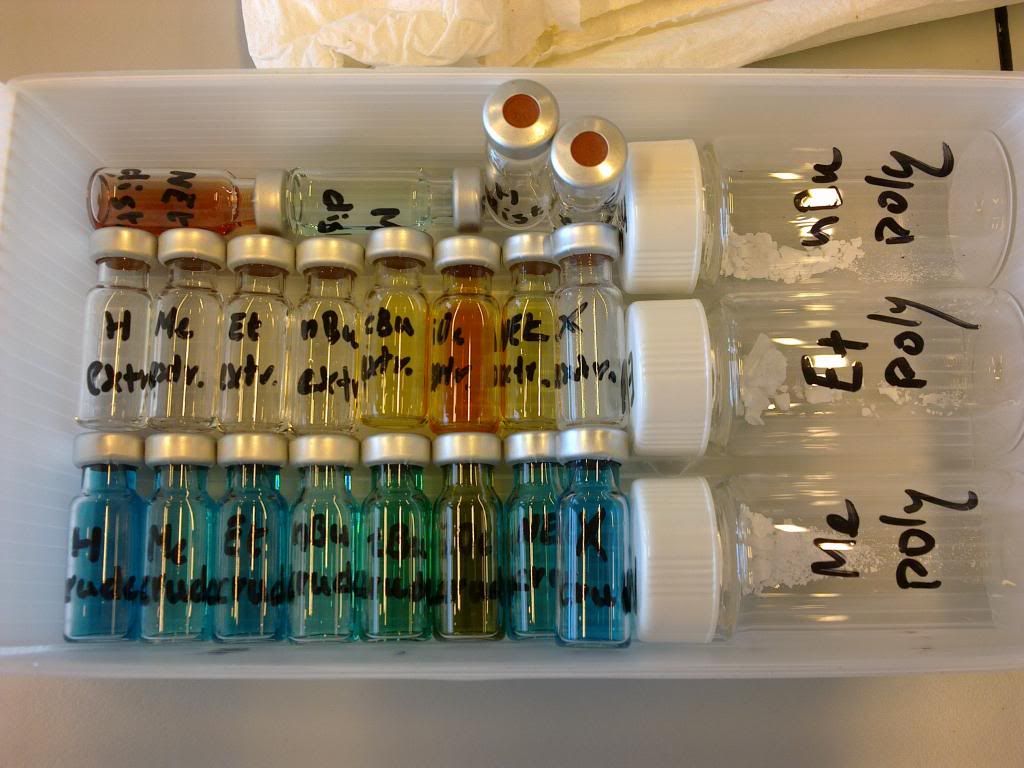
The resulting samples then looked like this:

and this:
Today; a part of my internship. I was making a series of organic polymers which I prepared in a copper solution.
The setup was like this:

The setup to the right (with the mechanical stirrer down the middle and the reflux bulb on the left) was used for the preparation of the crude product, the middle setup (with the makeshift infusion setup and massive conical flask) was used for aqueous workup and the one on the left (with the dropping funnel and reflux condenser) was for co-polymerisation.
The resulting samples then looked like this:

and this:

 Wizard
29 May 2013
Wizard
29 May 2013
Looks like you are going to some rather extraordinary lengths to make your own Smurf juice ... 
 Chyros
29 May 2013
Chyros
29 May 2013
Yes, in fact, everything I did during that internship was extremely blue, sometimes very prettily blue. Here's a few examples:
crude reaction mixture:

distillation extract:

concentrate of a crude:

crude reaction mixture:

distillation extract:

concentrate of a crude:



