AJ, on 16 Oct 2010, 3:42, said:
AJ, on 16 Oct 2010, 3:42, said:
 Grand Kapitan Camo, on 16 Oct 2010, 2:15, said:
Grand Kapitan Camo, on 16 Oct 2010, 2:15, said:
Actually, table salt is sodium merged with chlorine, making it stable. You do NOT want to put pure sodium on your french fries, especially if there greasy...
Almost complete fail in this statement. Chyros never said the sodium wasn't stable, he was just pointing out that the element is the same in both the pure metal, and the common table salt. the bonding with chlorine does produce the stability, however.
That's good, but it's actually the other way around - sodium (Na) is unstable versus oxidants like water, but sodium cation (Na
+) is perfectly stable - however not because it bonds with Cl
-. This is easily visible since Na
+ forms quite exothermically if you dissolve Na in water, ethanol, any simple alcohol, really. The fact this cation is stable allows it to form ionic bonds with for example Cl
- to form NaCl.
The reason Na
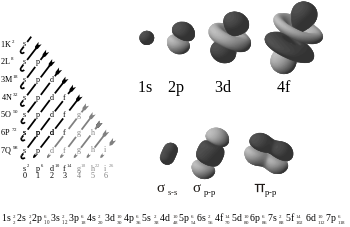
+ is stable and Na isn't, is because the electron configuration of Na is 1s
22s
22p
63s
1 - an oxidant will allow it to shed its single valence electron (3s
1) and form 1s
22s
22p
6 which is the electron configuration of neon, a noble gas. Noble gases have closed-shell configurations (valence electrons occupy all orbitals fully) and are energetically very stable - because of this, they are almost completely inert (and so is Na
+). The reason chlorine is so reactive is for the exact same but opposite reason - it likes to form Cl
- because this will change its configuration from 1s
22s
22p
63s
23p
5 to 1s
22s
22p
63s
23p
6 (absorbing an electron will allow it to fill up its single valence orbital hole) and form the electron configuration of argon which is also a noble gas. Such ionically stable species will readily combine to form NaCl.
Quote
And finally, putting pure sodium on greasy chips wouldn't be that much of a big deal - it reacts violently with water, and greasy chips are coated in oil, which is exactly what is used to keep sodium from becoming volatile (oil or kerosene). Sodium will react with water in the palm of your hand, however, but not to a degree by which you're likely to get more than slightly scalded - the entire metal needs to be contained in a reasonably large volumme of water to prove volatile on the levels that people expect of it.
That's indeed very true, but I think the saliva in your mouth will qualify for that

. You wouldn't enjoy the sensation in your mouth

. Grease on the chips wouldn't do much though.
Quote
Also, a quick point to note regards sodium form a biologist's perspective, you may want to check out this:
http://en.wikipedia....%2B/K%2B-ATPase - without this pump nerves would not function and we'd all be well and truly dead. Never underestimate the importance of an element, just because it looks nasty on the surface

Yes, very true. In fact, many powerful (nerve) toxins work by blocking sodium channels or pumps and this will kill you very quickly (black mamba, fugu, cone snails, blue-ringed octopus, poison dart frogs, Sydney funnel-web spiders, box jellyfish etc. all use venom that blocks or modifies these pumps or channels).
 AJ, on 16 Oct 2010, 3:42, said:
AJ, on 16 Oct 2010, 3:42, said: Grand Kapitan Camo, on 16 Oct 2010, 2:15, said:
Grand Kapitan Camo, on 16 Oct 2010, 2:15, said: