
Dauth's little corner of Science
#126
Posted 31 January 2009 - 21:53
Though, I'd like to put a question for Dauth:
What causes some elements to dissolve or react faster when in colder conditions. I suppose that it has some connection with inter-molecular hydrogen connections (don't know the correct english explanation for it, it's a link between atoms of water, if you know what I'm talking about)?
18.11.1991. REMEMBER VUKOVAR!
#127
Posted 31 January 2009 - 22:05
Reactions tend to be faster at higher temperatures since there are more interactions per second, and more molecules have enough energy to overcome the activation energy. As for faster when colder I'd like to see a specific example to help describe it.
Hope this helps.
#128
Posted 01 February 2009 - 00:08
When a capacitor is in the process of charging, labor(work?) is done by an external source - a battery of sorts, for example. When said force is terminated, charge separation persists and a certain amount of energy is stored, until the system is returned into equilibrium (joining the poles).
My question is: where is the energy actually being stored? Especially if the capacitor is divided by vacuum.
#129
Posted 01 February 2009 - 04:11
 Z_mann, on 31 Jan 2009, 19:08, said:
Z_mann, on 31 Jan 2009, 19:08, said:
When a capacitor is in the process of charging, labor(work?) is done by an external source - a battery of sorts, for example. When said force is terminated, charge separation persists and a certain amount of energy is stored, until the system is returned into equilibrium (joining the poles).
My question is: where is the energy actually being stored? Especially if the capacitor is divided by vacuum.
Energy is stored as electric potential energy in the Uniform Electric Field I believe. Energy doesn't require a material to be stored in. In fact, many capacitors use dielectric materials for higher capacitance and better structure I think. We'll have Dauth confirm this.
#130
Posted 11 March 2010 - 00:31
 Z_mann, on 1 Feb 2009, 1:08, said:
Z_mann, on 1 Feb 2009, 1:08, said:
When a capacitor is in the process of charging, labor(work?) is done by an external source - a battery of sorts, for example. When said force is terminated, charge separation persists and a certain amount of energy is stored, until the system is returned into equilibrium (joining the poles).
My question is: where is the energy actually being stored? Especially if the capacitor is divided by vacuum.
It's stored in the electrostatic fields that hold the electrons on the negatively charged side.

 19681107
19681107
#131
Posted 11 March 2010 - 09:30
#132
Posted 15 March 2010 - 08:50

 19681107
19681107
#133
Posted 15 March 2010 - 09:09
Phosphorous-239 (P-239) has never and I suspect will never be observed.
if you're after Plutonium, then I think that you should talk to real nuclear physicists. i would always recommend a moderator, just so you can control the reaction and prevent it going super critical.
#134
Posted 15 March 2010 - 09:26
 Dauth, on 15 Mar 2010, 10:09, said:
Dauth, on 15 Mar 2010, 10:09, said:
Phosphorous-239 (P-239) has never and I suspect will never be observed.
if you're after Plutonium, then I think that you should talk to real nuclear physicists. i would always recommend a moderator, just so you can control the reaction and prevent it going super critical.
Well, it's just that, purely speculating here, if given those conditions you CAN productively breed plutonium, I might have found a way someone could assemble a nuclear bomb. Not that I would ever approach that Darwin Award level stupidity.

 19681107
19681107
#135
Posted 15 March 2010 - 10:19
 The Machman, on 15 Mar 2010, 11:26, said:
The Machman, on 15 Mar 2010, 11:26, said:
 Dauth, on 15 Mar 2010, 10:09, said:
Dauth, on 15 Mar 2010, 10:09, said:
Phosphorous-239 (P-239) has never and I suspect will never be observed.
if you're after Plutonium, then I think that you should talk to real nuclear physicists. i would always recommend a moderator, just so you can control the reaction and prevent it going super critical.
Well, it's just that, purely speculating here, if given those conditions you CAN productively breed plutonium, I might have found a way someone could assemble a nuclear bomb. Not that I would ever approach that Darwin Award level stupidity.
Just from nuclear collision you'd probably not manage to make critical mass anyway, since you need about 10 kg = 41 moles = 2,5∙1025 atoms of Pu-239. Considering the chances of one nuclear collision happening I'd say that's not bloody likely
The brave hide behind technology. The stupid hide from it. The clever have technology, and hide it.
—The Book of Cataclysm


#136
Posted 14 May 2010 - 03:19
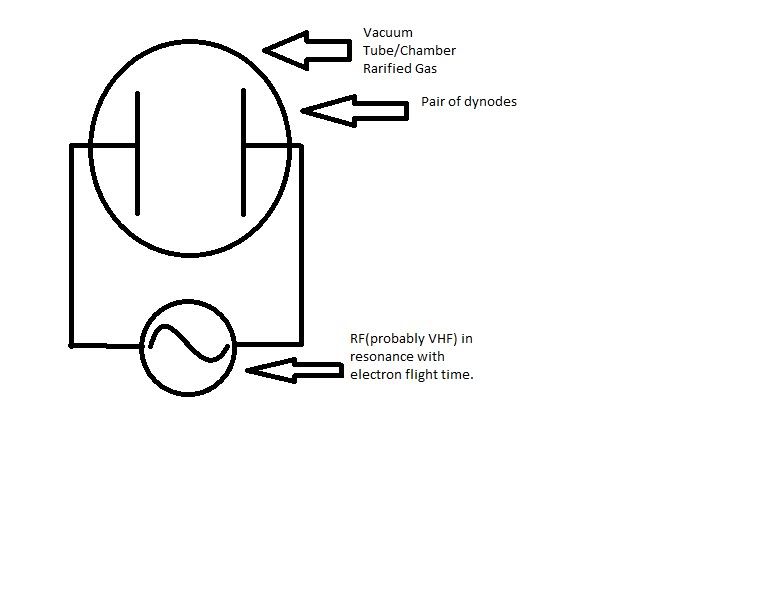
Let's say you have two dynodes in a vacuum tube, and you apply a RF signal to them so that they are 90 degrees out of phase, and then make this signal resonant with the electron flight time across the tube at whatever voltage the dynodes are charged to(let's say 20kV for the sake of debate). When the electrons slam into the dynode, they should release more electrons via secondary electron emission. Now this might seem like one of those really, really, really, "Well DUH" kind of questions, but when the secondary electrons are created does that cause the plate they are liberated from to have a higher positive potential due to lost electrons?
Edited by Dr. Strangelove, 14 May 2010 - 03:20.

 19681107
19681107
#137
Posted 06 July 2010 - 21:06
Emitting electrons will naturally make the dynode charged more positively compared to its state before the emission simply due to the decrease in electron number.
#138
Posted 20 September 2010 - 12:26
#139
Posted 20 September 2010 - 13:56
#140
Posted 20 September 2010 - 16:22
Edited by Sargeant Rho, 20 September 2010 - 16:22.
#141
Posted 20 September 2010 - 16:24
As you might have heard, electromagnetic waves exist only in discrete packages, so called photons. Every photon carries a discrete amount of energy defined by its frequency, E=hv. This means that electromagnetic waves of different frequency (and as such, wavelength) also have a proportionally different energy (inversely proportional to the wavelength). For example, gamma radiation has the highest energy per photon whereas radio waves carry only little energy per photon.
Now, the transparency/opacity of an object depends on what amounts of energy it can absorb. For example, electrons orbiting around an atom nucleus carry discrete amounts of energy based on their orbiting radius (similar to the photon's energy-frequency relation) while the radii themselves are also limited to discrete values - thus, an electron absorbing photons to "hop" onto a wider radius can only absorb photons of a specific, limited frequency range. This usually happens in the range of and around of the visible spectrum. It is also the reason why the transparency of a material can be used as a rough indicator for how well it serves as an electrical insulator (e.g. transparent materials have their electrons bound tightly).
Other frequencies are subject to absorption through other effects - for example, photons can be absorbed by atom nuclei directly or link into the natural Brownian oscillation of crystals. Also, many materials will have the individual effects work together - for example, a photon can loose energy by physically hitting a nucleus, then be absorbed by an electron due to its energy now matching the gap energy, then re-emitted by the electron jumping back to its initial radius and then absorbed by another material.
Edited by Golan, 20 September 2010 - 16:27.
#144
Posted 25 July 2017 - 07:16
Wish I could get your answer!
2 user(s) are reading this topic
0 members, 2 guests, 0 anonymous users

 This topic is locked
This topic is locked





