Chyros' chemistry thread
#101
Posted 06 December 2010 - 05:18
#102
Posted 06 December 2010 - 05:47
The brave hide behind technology. The stupid hide from it. The clever have technology, and hide it.
—The Book of Cataclysm


#103
Posted 06 December 2010 - 06:34
#104
Posted 06 December 2010 - 09:13
 Destiny, on 6 Dec 2010, 8:34, said:
Destiny, on 6 Dec 2010, 8:34, said:
The brave hide behind technology. The stupid hide from it. The clever have technology, and hide it.
—The Book of Cataclysm


#105
Posted 08 December 2010 - 19:29
TLC is one of the most basic means of analysing a reaction mixture. New reactions are generally monitored by TLC every x minutes to check their progression, and TLC functions as a rudimentary way of checking a compound's purity and to compare against other compounds to check what the impurities may be. It also paves the way for follow-up methods such as PLC, column chromatography etc.
TLC works by using a stationary phase, i.e. a powdery solid, through which the compounds may pass at variable speed. They are pulled through the solids by use of an eluent, a mixture of solvents with a specially designed polarity, that pulls the compounds you apply on the TLC paper to varying degrees. Using stronger eluents (more polar mixtures, such as 5% HCOOH in MeOH, to name a very polar one) will pull compounds along more strongly, while weaker eluents (very apolar ones, for example 2% DCM in hexane, to name a very apolar one) will make compounds move very little. Different compounds will, due to their varying polarity, move through the stationary phase with different speeds, and through this, separation is achieved, showing you how many compounds are (at least) present in the mixture, and if you have a reference, you can identify if a certain compound is in the mixture or not.
There are many subtleties, tips, tricks and traps to TLC, which despite seemingly a very simple analytical method, will catch the unwary synthetic organic chemist off guard. In the interests of time and accessibility of the information, I didn't cover these aspects.
More to come on subjects related to TLC.
The brave hide behind technology. The stupid hide from it. The clever have technology, and hide it.
—The Book of Cataclysm


#106
Posted 08 December 2010 - 20:04
So what would use does TLC have in a real life situation?

F O R T H E N S

#107
Posted 08 December 2010 - 21:03
 TheDR, on 8 Dec 2010, 22:04, said:
TheDR, on 8 Dec 2010, 22:04, said:
So what would use does TLC have in a real life situation?

The brave hide behind technology. The stupid hide from it. The clever have technology, and hide it.
—The Book of Cataclysm


#108
Posted 08 December 2010 - 21:10
 Chyros, on 8 Dec 2010, 22:03, said:
Chyros, on 8 Dec 2010, 22:03, said:
 TheDR, on 8 Dec 2010, 22:04, said:
TheDR, on 8 Dec 2010, 22:04, said:
So what would use does TLC have in a real life situation?
I'd like to point out that electrophoresis was first used to run prenatal diagnoses on fetuses in order to determine whether they had any hereditary disease
#109
Posted 09 December 2010 - 00:19
...say, do you have problems doing the videos with other dudes around?
#110
Posted 09 December 2010 - 06:08
 Destiny, on 9 Dec 2010, 2:19, said:
Destiny, on 9 Dec 2010, 2:19, said:
...say, do you have problems doing the videos with other dudes around?
About the second thing; what do you mean?
The brave hide behind technology. The stupid hide from it. The clever have technology, and hide it.
—The Book of Cataclysm


#111
Posted 09 December 2010 - 06:19
#112
Posted 09 December 2010 - 06:25
 Destiny, on 9 Dec 2010, 8:19, said:
Destiny, on 9 Dec 2010, 8:19, said:
The brave hide behind technology. The stupid hide from it. The clever have technology, and hide it.
—The Book of Cataclysm


#113
Posted 09 December 2010 - 08:27
 Chyros, on 9 Dec 2010, 17:25, said:
Chyros, on 9 Dec 2010, 17:25, said:
 Destiny, on 9 Dec 2010, 8:19, said:
Destiny, on 9 Dec 2010, 8:19, said:
Keep the ruined takes so we can hear you telling them to shut up.
------------------------------------------------------------
----------------------------------------
------------------------------------------------------------
----------------------------------------
--------------------
The name's Bond.
Covalent Bond.
#114
Posted 09 December 2010 - 08:30
#115
Posted 09 December 2010 - 13:28
 deltaepsilon, on 9 Dec 2010, 10:27, said:
deltaepsilon, on 9 Dec 2010, 10:27, said:
 Chyros, on 9 Dec 2010, 17:25, said:
Chyros, on 9 Dec 2010, 17:25, said:
 Destiny, on 9 Dec 2010, 8:19, said:
Destiny, on 9 Dec 2010, 8:19, said:
Keep the ruined takes so we can hear you telling them to shut up.
Besides, I don't know how to do video editing so it would be too much of a bother to in the first place
Edited by Chyros, 09 December 2010 - 13:30.
The brave hide behind technology. The stupid hide from it. The clever have technology, and hide it.
—The Book of Cataclysm


#116
Posted 11 January 2011 - 20:54
Column chromatography is a separation method based on the same principles as thin-layer chromatography; see my previous video on this subject for more information: http://www.youtube.c...h?v=1rt25dy_hxw
Note that unlike TLC, column chromatography is not an analytical method, but a preparatory one; not qualitative but quantitative. You use it to purify a mixture, not determine what it's composed of. Nonetheless, column chromatography is practically always accompanied by TLC'ing the fractions to determine the compounds in them and therefore, their composition and purity.
The method employs a gel of a stationary phase in an eluent through which the compounds in a mixture may pass at variable speed. They are pulled through the solids by use of more eluent sitting on top of the stationary phase, with the polarity and properties of the eluent designed around separating any impurities as well as possible from your target compound. The eluent (with any compounds in solution) drips out at the tap at the bottom of the column and this is collected in small containers as fractions of the column. This allows you to test all the fractions for which compounds are in them, and when you know this, you can take the fractions which contain ONLY your product and discard and that contain impurities. Combination of these pure fractions and consecutive removal of the solvent will then yield your product in a pure fashion.
Thanks to TheDR for rotating the video for me: it was originally turned 90 degrees from what it is now.
The brave hide behind technology. The stupid hide from it. The clever have technology, and hide it.
—The Book of Cataclysm


#117
Posted 12 January 2011 - 01:45

#118
Posted 12 January 2011 - 07:22
The brave hide behind technology. The stupid hide from it. The clever have technology, and hide it.
—The Book of Cataclysm


#119
Posted 12 January 2011 - 07:35
 Chyros, on 11 Jan 2011, 15:54, said:
Chyros, on 11 Jan 2011, 15:54, said:
*video*
Column chromatography is a separation method based on the same principles as thin-layer chromatography; see my previous video on this subject for more information: http://www.youtube.c...h?v=1rt25dy_hxw
Note that unlike TLC, column chromatography is not an analytical method, but a preparatory one; not qualitative but quantitative. You use it to purify a mixture, not determine what it's composed of. Nonetheless, column chromatography is practically always accompanied by TLC'ing the fractions to determine the compounds in them and therefore, their composition and purity.
The method employs a gel of a stationary phase in an eluent through which the compounds in a mixture may pass at variable speed. They are pulled through the solids by use of more eluent sitting on top of the stationary phase, with the polarity and properties of the eluent designed around separating any impurities as well as possible from your target compound. The eluent (with any compounds in solution) drips out at the tap at the bottom of the column and this is collected in small containers as fractions of the column. This allows you to test all the fractions for which compounds are in them, and when you know this, you can take the fractions which contain ONLY your product and discard and that contain impurities. Combination of these pure fractions and consecutive removal of the solvent will then yield your product in a pure fashion.
Thanks to TheDR for rotating the video for me: it was originally turned 90 degrees from what it is now.
So can you customize the eluent to the compound you want to separate by adjusting the polarity/other properties of it?

AJ is responsible for this signature masterpiece... if you see him, tell him I say thanks.

#120
Posted 12 January 2011 - 08:58
 Ghostrider, on 12 Jan 2011, 9:35, said:
Ghostrider, on 12 Jan 2011, 9:35, said:
 Chyros, on 11 Jan 2011, 15:54, said:
Chyros, on 11 Jan 2011, 15:54, said:
*video*
Column chromatography is a separation method based on the same principles as thin-layer chromatography; see my previous video on this subject for more information: http://www.youtube.c...h?v=1rt25dy_hxw
Note that unlike TLC, column chromatography is not an analytical method, but a preparatory one; not qualitative but quantitative. You use it to purify a mixture, not determine what it's composed of. Nonetheless, column chromatography is practically always accompanied by TLC'ing the fractions to determine the compounds in them and therefore, their composition and purity.
The method employs a gel of a stationary phase in an eluent through which the compounds in a mixture may pass at variable speed. They are pulled through the solids by use of more eluent sitting on top of the stationary phase, with the polarity and properties of the eluent designed around separating any impurities as well as possible from your target compound. The eluent (with any compounds in solution) drips out at the tap at the bottom of the column and this is collected in small containers as fractions of the column. This allows you to test all the fractions for which compounds are in them, and when you know this, you can take the fractions which contain ONLY your product and discard and that contain impurities. Combination of these pure fractions and consecutive removal of the solvent will then yield your product in a pure fashion.
Thanks to TheDR for rotating the video for me: it was originally turned 90 degrees from what it is now.
So can you customize the eluent to the compound you want to separate by adjusting the polarity/other properties of it?
If you want to catch multiple dots, for example one that elutes very quickly and one that elutes very slowly, you can use a gradient in your eluent: start off with a very apolar eluent such as Et2O in hexane and when you have that compound off the column, you slowly add a higher concentration of a very polar eluent such as MeOH or HCOOH.
Sometimes, like in my case, the column actually reacts with your compound. Silica, the most often-used stationary phase, is acidic and can therefore form hydrogen bonds with certain compounds (which means the compound will be bound very strongly to the column and regardless of how polar your solvent is, you may not get it off your column at all) or it may protonate your compound which can sometimes destroy the compound. To prevent this, you can for example use a few percents of Et3N in your eluent, which is a weak, non-nucleophilic base (which happens to reek of semen :s) that neutralises the acidity of your column. In my case, I use complicated phosphine oxides which are impossible to get off without some base as I found the oxides are retained very strongly retained by the acidic silica:
Si-O-H ..... O=P-R
An alternative would be to use MeOH in the eluent (though this makes my compounds elute too quickly):
MeO-H ..... O=P-R
The brave hide behind technology. The stupid hide from it. The clever have technology, and hide it.
—The Book of Cataclysm


#121
Posted 15 January 2011 - 08:19

Now here's a column I ran once which had an interesting effect:

The reaction mixture I tried to purify here turned out to contain all colours and separating them via column chromatography produced a very nice rainbow effect in my column.
The brave hide behind technology. The stupid hide from it. The clever have technology, and hide it.
—The Book of Cataclysm


#122
Posted 15 January 2011 - 10:28
...but that's pretty chromatography. I wonder if you shined UV or something light on it, what'll happen...
#123
Posted 15 January 2011 - 10:55
 Destiny, on 15 Jan 2011, 12:28, said:
Destiny, on 15 Jan 2011, 12:28, said:
Quote
A friend of mine in FOC works with pyrene (
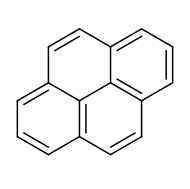 ) derivatives. These molecules are highly UV-active because of their extensive delocalised pi-electron system which allows them to reorganise electrons and emit photons in the form of visible light and even the tiniest contamination with them makes something very UV-active. He ran a column on them a while ago and I took some pictures. Because his molecules are so UV-active this allows him to actually see the compounds move through the column using a handheld UV lamp.
) derivatives. These molecules are highly UV-active because of their extensive delocalised pi-electron system which allows them to reorganise electrons and emit photons in the form of visible light and even the tiniest contamination with them makes something very UV-active. He ran a column on them a while ago and I took some pictures. Because his molecules are so UV-active this allows him to actually see the compounds move through the column using a handheld UV lamp. This is what the column looked like:

And this is what the eluent looked like; the minor contamination from his compounds was enough to make the entire eluent body light up like a Christmas tree:

Edited by Chyros, 15 January 2011 - 11:08.
The brave hide behind technology. The stupid hide from it. The clever have technology, and hide it.
—The Book of Cataclysm


#124
Posted 15 January 2011 - 11:14

No really, it's cool! The first one looks...really...well...hmm...how do I phrase it...
#125
Posted 15 January 2011 - 14:59
The brave hide behind technology. The stupid hide from it. The clever have technology, and hide it.
—The Book of Cataclysm


1 user(s) are reading this topic
0 members, 1 guests, 0 anonymous users