Chyros' chemistry thread
#126
Posted 30 January 2011 - 11:15
The rotavap is a common piece of machinery used to gently boil off solvents under reduced pressure. Using a membrane pump to create a vacuum will make solvents boil off faster, and rotating the flask gives you the advantage of creating more area for the solvent to evaporate off from as well as preventing superheating (which will result in the solvent suddenly boiling extremely violently). The rotavap combines all these with the option of using a water bath to keep the flask on room temperature (normally evaporation of solvents inside will quickly cool down the flask since evaporation is an endothermic process) or allows heating to speed up evaporation.
If you contaminate the rotavap with solvents that are hard to get rid of (such as water) or traces of compound (for example low-boiling organic compounds), you need to clean it to prevent reflux of these materials to contaminate the compounds of whoever uses the rotavap next. To do so, you can disassemble the machine and very manually clean it but this takes a disproportionate amount of effort, or you can use a splashing tube which is a bent glass tube with a socket at the end. If you connect this to the rotavap, fill it up with some clean solvent that evaporates easily, such as acetone, put on vacuum and block then end of the tube, the pressure in the rotavap will lower, and if you remove the blockade, the outside air will quickly rush in and push the solvent through the system, splashing it clean in an easy and convenient way.
I hate how nasal my voice sounds in these videos >.> .
The brave hide behind technology. The stupid hide from it. The clever have technology, and hide it.
—The Book of Cataclysm


#127
Posted 30 January 2011 - 12:08

AJ is responsible for this signature masterpiece... if you see him, tell him I say thanks.

#128
Posted 30 January 2011 - 12:15
They way you pulled out that flask from nowhere and kept it looked just like in FPSes
#129
Posted 30 January 2011 - 12:25
 Ghostrider, on 30 Jan 2011, 14:08, said:
Ghostrider, on 30 Jan 2011, 14:08, said:
 Destiny, on 30 Jan 2011, 14:15, said:
Destiny, on 30 Jan 2011, 14:15, said:
Quote
The brave hide behind technology. The stupid hide from it. The clever have technology, and hide it.
—The Book of Cataclysm


#130
Posted 09 July 2011 - 16:23
Synthesis sometimes needs to be conducted under reduced temperature. This can be because, for example, the reaction may proceed too fast to be selective at room temperature, or it may be too vigorous or exothermic and explode. As such, many studied reactions are optimised to a certain temperature.
The three most easily attainable temperatures are 0 deg C (cool with ice), -15 C (ice in salt water), -80 C (dry ice) and -200 C (liquid nitrogen). However, sometimes you need a temperature that may not be very straightforward to maintain, such as -100 C. Using certain liquids provides a way to maintain temperatures like this - in the case of -100 C, it is liquid nitrogen in methanol, as is what happens here. Methanol has a melting point very close to -100 C and liquid nitrogen is cold enough to get it to that temperature.
The methanol bath is put into place, and a dewar flask with liquid nitrogen is poured out slowly into it. As the liquid nitrogen comes closer to the surface of the (room temperature and therefore over 200 degrees hotter) methanol, the nitrogen starts to boil violently, splashing methanol everywhere. The nitrogen evaporates so fast that it will create a cushion on nitrogenous steam which prevents the nitrogen from touching the methanol for a while (this is called the Leidenfrost effect and it's also the reason you can touch liquid nitrogen or "drink" it - the temperature of your body is enough to evaporate it before it touches you and shield you from the liquids) and you can see this as a bubbling mass on top of the methanol. After a while, the methanol has lost so much of its heat to the nitrogen that it can't boil the nitrogen very fast anymore, so the nitrogen will start touching the methanol's surface. You can recognise this by the steam becoming less dense and less white. It will then form small lumps of solid methanol, and some stirring will then make everything solidify instantly.
The brave hide behind technology. The stupid hide from it. The clever have technology, and hide it.
—The Book of Cataclysm


#131
Posted 09 July 2011 - 21:32
#132
Posted 09 July 2011 - 23:29
With a bit of luck I'll do an interesting distillation soon which I predict will produce a dense, intensely blue foam
The brave hide behind technology. The stupid hide from it. The clever have technology, and hide it.
—The Book of Cataclysm


#133
Posted 10 July 2011 - 00:01
What was missing from my life has been dense, blue foam.
Edited by Alias, 10 July 2011 - 00:01.

#134
Posted 10 July 2011 - 00:09
 Alias, on 10 Jul 2011, 2:01, said:
Alias, on 10 Jul 2011, 2:01, said:
What was missing from my life has been dense, blue foam.
But it's not too late to turn around and join the ways of science!
The brave hide behind technology. The stupid hide from it. The clever have technology, and hide it.
—The Book of Cataclysm


#135
Posted 10 July 2011 - 04:45
#136
Posted 10 July 2011 - 09:40
We do have ultra-pure silica, though. The material it's made out of.
The brave hide behind technology. The stupid hide from it. The clever have technology, and hide it.
—The Book of Cataclysm


#137
Posted 10 July 2011 - 11:30
#138
Posted 03 August 2011 - 15:59


The brave hide behind technology. The stupid hide from it. The clever have technology, and hide it.
—The Book of Cataclysm


#139
Posted 03 August 2011 - 16:03
#140
Posted 03 August 2011 - 17:06

F O R T H E N S

#141
Posted 03 August 2011 - 18:42
#142
Posted 03 August 2011 - 21:50
#143
Posted 03 August 2011 - 22:32
 Destiny, on 03 August 2011 - 21:50, said:
Destiny, on 03 August 2011 - 21:50, said:
So you'd definitely have to buy a new shirt for them
I wasted about three shirts during my first year by simply being around it without proper lab coat protection - small amounts of vapours are enough to inconspicuously make small holes in your clothes everywhere until you take a look at your clothes and find they are actually completely falling apart.
Edited by Chyros, 03 August 2011 - 22:34.
The brave hide behind technology. The stupid hide from it. The clever have technology, and hide it.
—The Book of Cataclysm


#144
Posted 04 August 2011 - 04:28
#147
Posted 04 August 2011 - 08:00
 Wizard, on 04 August 2011 - 07:44, said:
Wizard, on 04 August 2011 - 07:44, said:
The brave hide behind technology. The stupid hide from it. The clever have technology, and hide it.
—The Book of Cataclysm


#148
Posted 26 May 2013 - 21:10
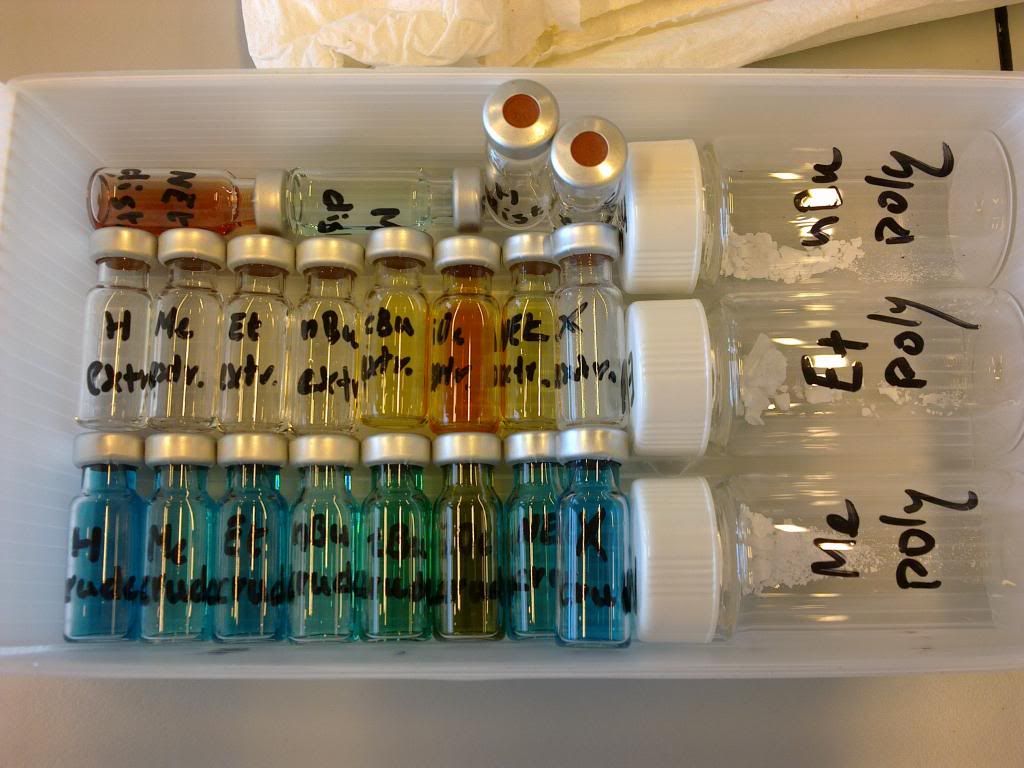
Today; a part of my internship. I was making a series of organic polymers which I prepared in a copper solution.
The setup was like this:

The setup to the right (with the mechanical stirrer down the middle and the reflux bulb on the left) was used for the preparation of the crude product, the middle setup (with the makeshift infusion setup and massive conical flask) was used for aqueous workup and the one on the left (with the dropping funnel and reflux condenser) was for co-polymerisation.
The resulting samples then looked like this:

and this:
The brave hide behind technology. The stupid hide from it. The clever have technology, and hide it.
—The Book of Cataclysm


#149
Posted 29 May 2013 - 10:40
#150
Posted 29 May 2013 - 18:00
crude reaction mixture:

distillation extract:

concentrate of a crude:

The brave hide behind technology. The stupid hide from it. The clever have technology, and hide it.
—The Book of Cataclysm


1 user(s) are reading this topic
0 members, 1 guests, 0 anonymous users