Chyros' chemistry thread
#26
Posted 28 September 2010 - 14:36
I also have seen the blue one somewhere before, but I can't recall it.

NProject Mod -- Recolonize -- Tidal Wars
#28
Posted 28 September 2010 - 21:27
 scope, on 28 Sep 2010, 15:23, said:
scope, on 28 Sep 2010, 15:23, said:
Is the black precipitate Iron Sulfide? Is the first one Methyl Red?
-nitrate-trihydrate-sample.jpg/200px-Copper(II)-nitrate-trihydrate-sample.jpg)
it's not a shade of green. This compound is darker and clearly has a bit of green in it.
The black stuff isn't iron sulfide, which is dark brown. This compound is a very very very dark purple - so dark that it appears black under most circumstances. In terms of colour it's probably the compound with the most concentrated colour I know of. Solutions of this are very strongly coloured even in extreme dilution.
The red one is indeed methyl red, correct
Quote
 Mbob61, on 28 Sep 2010, 16:55, said:
Mbob61, on 28 Sep 2010, 16:55, said:
If not, i would guess something like Calcium Carbonate.
Also, bad times, i have left my old chem books at home so all this will be from memory
Mike
Edited by Chyros, 28 September 2010 - 21:37.
The brave hide behind technology. The stupid hide from it. The clever have technology, and hide it.
—The Book of Cataclysm


#29
Posted 29 September 2010 - 09:54
Anyway for the last one I was thinking about is oxidised magnesium, but it doesn't really make sense to keep that stuff around I guess. Since you've already spoiled that its a potassium salt which would make me guess potassium chloride though I have never seen it in such crystalized form.
Edited by Shirou, 29 September 2010 - 10:02.

#30
Posted 29 September 2010 - 10:02
#31
Posted 29 September 2010 - 12:42
------------------------------------------------------------
----------------------------------------
------------------------------------------------------------
----------------------------------------
--------------------
The name's Bond.
Covalent Bond.
#32
Posted 29 September 2010 - 19:30
 Shirou, on 29 Sep 2010, 11:54, said:
Shirou, on 29 Sep 2010, 11:54, said:
Anyway for the last one I was thinking about is oxidised magnesium, but it doesn't really make sense to keep that stuff around I guess. Since you've already spoiled that its a potassium salt which would make me guess potassium chloride though I have never seen it in such crystalized form.
 Destiny, on 29 Sep 2010, 12:02, said:
Destiny, on 29 Sep 2010, 12:02, said:
 deltaepsilon, on 29 Sep 2010, 14:42, said:
deltaepsilon, on 29 Sep 2010, 14:42, said:
The brave hide behind technology. The stupid hide from it. The clever have technology, and hide it.
—The Book of Cataclysm


#33
Posted 29 September 2010 - 22:58
On the SF6 well yeah I guess its quite expensive. May also be better to keep it at bay seeing how the shit is thousands of times more powerful a greenhouse gas than CO2. Dont want anyone on your roof after posting such a video xD.
Edited by Shirou, 29 September 2010 - 23:00.

#34
Posted 30 September 2010 - 02:21
#35
Posted 30 September 2010 - 06:44
Quote
Quote
Hmmm, yeah, seems about right
 Destiny, on 30 Sep 2010, 4:21, said:
Destiny, on 30 Sep 2010, 4:21, said:
To give some idea of the pricings:
He 57L canister, lowest purity - €190
SF6 227 g canister - €409
Perhaps if I look at the lab supervisor nicely I'll get some helium, got SF6 isn't gonna happen if I'm not gonna do a reaction with it
The brave hide behind technology. The stupid hide from it. The clever have technology, and hide it.
—The Book of Cataclysm


#36
Posted 30 September 2010 - 21:39
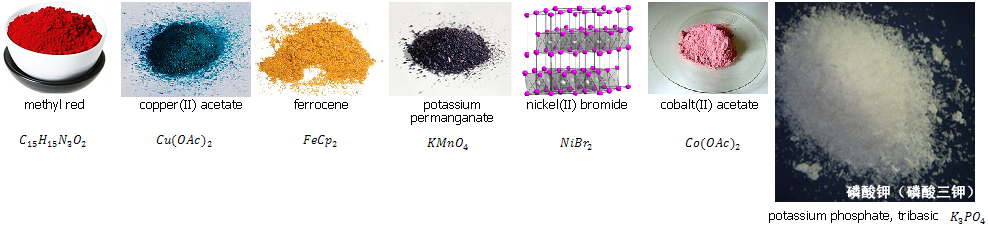
Methyl red was guessed correctly - it is a commonly used pH indicator when in solution, which is red in acidic and yellow in basic environments. The solid itself is also extremely red - such a pronounced colour is typical for azo dyes like this.
Copper acetate's bluish colour easily gives away that it's a copper compound as you guys quickly picked up, too. The acetate salt is IMO one of the most beautifully coloured copper compounds with its deep blue-green colour.
Ferrocene is the quintessential orange compound - orange solids are fairly rare, but this one is quite well-known and cheap. It's of high relevance in organometallic chemistry because it has very "standard" properties - a schoolbook example in OrgMetChem.
Potassium permanganate is one of the most well-known chemical compounds, since everyone has to to titration in high school and the titrant is almost invariably a solution of this. Even very dilute solutions have a deep purple colour, and the solid is so dark purple that it appears black. I'm sure a lot of you secretly knew this one
Nickel bromide is the hardest to guess here. I couldn't find a picture that quickly, but it is a yellow-ochre compound, and as you can see, it appears speckled with light green.
Cobalt acetate - powdered in the pic, which belies its more crystalline colour - has a perhaps rather surprising colour for cobalt, which is traditionally more associated with blue. In truth, cobalt takes on quite a few colours, the red-pink colour being quite unique to cobalt (though cobalt chloride looks almost identical).
Tripotassium phosphate (pic not shrunk to show the crystal structure) is a fairly boring potassium salt, like pretty much any potassium salt. It's just very white and has a needley crystal structure.
I'm letting the question of why the NiBr2 is speckled, stand, though. I'm sure you guys can work it out
The brave hide behind technology. The stupid hide from it. The clever have technology, and hide it.
—The Book of Cataclysm


#37
Posted 01 October 2010 - 10:17
------------------------------------------------------------
----------------------------------------
------------------------------------------------------------
----------------------------------------
--------------------
The name's Bond.
Covalent Bond.
#39
Posted 04 October 2010 - 18:50
 Chyros, on 29 Sep 2010, 0:17, said:
Chyros, on 29 Sep 2010, 0:17, said:
And I wasn't lying - I present you my vid on aqua regia ^^ .
In case you are wondering, aqua regia is a mixture of nitric and hydrochloric acid. Th resulting acid is very powerful - even experienced lab practitioners avoid using this compound. Well, inorganic chemists do - for us synthetic organic chemists, it's not that spectacularly dangerous at all
The brave hide behind technology. The stupid hide from it. The clever have technology, and hide it.
—The Book of Cataclysm


#40
Posted 05 October 2010 - 00:50
Also, what neutralizes bacon oil? Pouring it down the sink is rather...bad.
Edited by Destiny, 05 October 2010 - 15:46.
#41
Posted 05 October 2010 - 16:14


Awesome radio
Quote
#42
Posted 05 October 2010 - 17:39
 Destiny, on 5 Oct 2010, 2:50, said:
Destiny, on 5 Oct 2010, 2:50, said:
It's also probably better to put it in the toilet than in the sink
NB: I should add that you shouldn't pour washing petrol or turpentine or stuff like that down the drain either
 ΓLambdaRhoTauThetaGamma, on 5 Oct 2010, 18:14, said:
ΓLambdaRhoTauThetaGamma, on 5 Oct 2010, 18:14, said:
Edited by Chyros, 05 October 2010 - 17:42.
The brave hide behind technology. The stupid hide from it. The clever have technology, and hide it.
—The Book of Cataclysm


#43
Posted 05 October 2010 - 18:22
#44
Posted 05 October 2010 - 22:02
 Destiny, on 5 Oct 2010, 20:22, said:
Destiny, on 5 Oct 2010, 20:22, said:
Edited by Chyros, 05 October 2010 - 22:02.
The brave hide behind technology. The stupid hide from it. The clever have technology, and hide it.
—The Book of Cataclysm


#45
Posted 07 October 2010 - 16:15
Nickel bromide or NiBr2 is a so-called hygroscopic material. This means that it can pull moisture from the air and incorporate this into its crystal structure. Many many many everyday compounds are hygroscopic: kitchen salt for example. this will then form lumps and clog up your salt pot, so many people put in, for example, grains of rice. Rice is also hygroscopic and in fact more so than kitchen salt, preventing the salt from forming lumps.
In the case of NiBr2 the absorbed moisture (three molecules of water per unit of NiBr2) will actually change the molecular properties: you will get NiBr2.3H2O which is not ochre but light green. So the green speckles are NiBr2.3H2O formed as a result of the anhydrous (ochre) NiBr2 being exposed to the moisture in the atmosphere for a while. If you left the container open for long enough, the whole top would turn green.
Edited by Chyros, 07 October 2010 - 16:17.
The brave hide behind technology. The stupid hide from it. The clever have technology, and hide it.
—The Book of Cataclysm


#47
Posted 14 October 2010 - 22:27
#48
Posted 14 October 2010 - 23:03
 Grand Kapitan Camo, on 15 Oct 2010, 0:27, said:
Grand Kapitan Camo, on 15 Oct 2010, 0:27, said:
I can explain why they are so reactive though. I'll do that tomorrow as well, I'm really tired atm.
Here's a vid showing the effects of small amounts of alkali metals in water. They're all really reactive but some obviously more so than others. This particular vid is a lot more truthful than others you'll find - the well-known Brainiac one for example was faked.
One thing though: the sodium in table salt is sodium, just not elemental (metallic) sodium. The element is the same, the sodium in NaCl isn't a "different" sodium from just metallic sodium
The brave hide behind technology. The stupid hide from it. The clever have technology, and hide it.
—The Book of Cataclysm


#49
Posted 16 October 2010 - 01:15
EDIT: French fries? Or French Fires?
Edited by Grand Kapitan Camo, 16 October 2010 - 01:15.
#50
Posted 16 October 2010 - 01:42
 Grand Kapitan Camo, on 16 Oct 2010, 2:15, said:
Grand Kapitan Camo, on 16 Oct 2010, 2:15, said:
Almost complete fail in this statement. Chyros never said the sodium wasn't stable, he was just pointing out that the element is the same in both the pure metal, and the common table salt. the bonding with chlorine does produce the stability, however. And finally, putting pure sodium on greasy chips wouldn't be that much of a big deal - it reacts violently with water, and greasy chips are coated in oil, which is exactly what is used to keep sodium from becoming volatile (oil or kerosene). Sodium will react with water in the palm of your hand, however, but not to a degree by which you're likely to get more than slightly scalded - the entire metal needs to be contained in a reasonably large volumme of water to prove volatile on the levels that people expect of it.
Also, a quick point to note regards sodium form a biologist's perspective, you may want to check out this: http://en.wikipedia....%2B/K%2B-ATPase - without this pump nerves would not function and we'd all be well and truly dead. Never underestimate the importance of an element, just because it looks nasty on the surface
2 user(s) are reading this topic
0 members, 2 guests, 0 anonymous users